
Haloalkanes
Definition of Haloalkanes:
Haloalkanes are chemical compounds in which one or more of the hydrogen atoms in an alkane have been replaced by halogen atoms (usually one or more of fluorine, chlorine, bromine or iodine).
As also applies to alkanes, haloalkanes are saturated organic compounds, meaning that all of the chemical bonds attaching the atoms within the molecule are single bonds.
Each carbon atom forms 4 bonds (either with other carbon atoms or with hydrogen or halogen atoms). Each hydrogen and halogen atom is connected to a single carbon atom.
Haloalkanes are also known as halogenalkanes and (less commonly) as alkyl halides.
More about Haloalkanes
Alkanes are one of the simplest types of organic compounds and consist only of carbon and hydrogen atoms linked together exclusively by single bonds (i.e. alkanes are saturated hydrocarbons). Haloalkanes are similar to alkanes but one or more of the hydrogen atoms in the corresponding alkane is replaced by a halogen atom(s) in the haloalkane.
What are halogens ?
Halogens are the elements in Group 7 (Group VII) of the periodic table, specifically: Fluorine (F), Chlorine (Cl), Bromine (Br), Iodine (I) and Astatine (At). The halogens most commonly found in, and of greatest importance in organic chemistry, are fluorine, chlorine, bromine and iodine.
Is there a general formula for haloalkanes ?
A simple general formula that describes many (but not all) of the haloalkanes usually included in basic chemistry courses is :
CnH2n+1X
where the letter n represents the number of carbon atoms in each molecule of the compound and the letter X represents a particular halogen atom. An example of a real chemical described by this formula is fluoromerhane (also known as methyl fluoride), whose molecules have just one carbon atom (so n=1) and includes the halogen fluorine (so X=F), hence it has the chemical formula CH3F.
Examples of Haloalkanes
The simplest way to convey what haloalkanes are and how they differ from alkanes is using molecular diagrams of some simple haloalkanes together with the corresponding alkane, for comparison. (The names of the chemicals whose structures are shown below are explained further down this page.)
Example of Haloalkane |
Corresponding Alkane |
||||
1 |
Fluoromethane |
|
Methane |
|
|
2 |
Bromoethane |
|
Ethane |
|
|
3 |
Chloropropane |
|
Propane |
|
|
4 |
Iodobutane |
|
Butane |
|
|
Example of Haloalkane |
Corresponding Alkane |
||
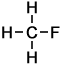
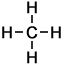
1 |
Fluoromethane |
Methane |
|
 |
 |
||
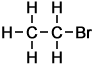
2 |
Bromoethane |
Ethane |
|
 |
 |
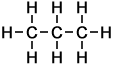
||
3 |
Chloropropane |
Propane |
|
 |
 |
||
4 |
Iodobutane |
Butane |
|
 |
 |
||
The above molecular structures have been drawn was simply as possible.
In all cases it is easy to see that the hydrogen atom represented by the H on the extreme right-hand-side of the alkane on the right has been replaced by the halogen atom in each of the haloalkanes on the left. As indicated by these examples, simple haloalkanes are named according to the specific halogen element (F, Cl, Br or I) in the molecule and the number of carbon atoms (using the same system of naming as applies to alkanes). More information about structures, names and classification of haloalkanes follows below.
The simple molecular structures shown above all represent the halogen atoms as being located on the right-hand-side of the molecule. This is just to make clear the pattern and difference between these haloalkanes and their corresponding linear alkanes. In the case of halomethanes and haloethanes, all the possible positions at which the halogen atom may be attached to the rest of the molecule are equivalent, that is - there is only one structural isomer of halomethanes and haloethanes.
This is shown in the following diagrams of the possible ways of drawing the four halomethane molecules CH3F, CH3Cl, CH3Br and CH3I.
Name of Halomethane |
Four different ways to represent exactly the same molecular structure: |
fluoromethane CH3F |
 |
chloromethane CH3Cl |
 |
bromomethane CH3Br |
 |
iodomethane CH3I |
 |
- Fluoromethane CH3F

- Chloromethane CH3Cl

- Bromomethane CH3Br

- Iodomethane CH3I

However, in the cases of the larger haloalkanes, different chemicals result from arranging the atoms in different ways.
The following diagrams show the examples of the two structural isomers of bromopropane:
Example 1 : 6 different ways to draw 1-bromopropane |
|
 |
|
Example 2 : 2 different ways to draw 2-bromopropane |
|
 |
|
Example 1 :
6 different ways to draw 1-bromopropane
Example 2 :
2 different ways to draw 2-bromopropane
This simple example shows that it matters exactly how the atoms that form haloalkane molecules are arranged.
The simplest examples of structural isomerism of haloalkanes are the halopropanes, of which bromopropane (above) is an example. The larger the haloalkane molecule, the more different ways in which the atoms can be arranged to form molecules.
Classification of Haloalkanes
There are two main ways of classifying haloalkanes.
They are, according to:
- the type of halogen e.g. fluorine, chlorine, bromine and iodine, or
- the structure of the molecules, i.e. the position of the carbon atom(s) to which the halogen or halogens are attached.
1. Haloalkanes by Type of Halogen
Haloalkanes can be grouped, named, and described according to the particular halogen that has replaced a hydrogen atom in the corresponding alkane:
Halogen
Generic name of organic compounds that include this halogen (as a 'functional group')
Examples of Haloalkanes:
(simple examples, based on descriptions above)
Fluorine (F)
organofluorine compounds
Chlorine (Cl)
organochlorine compounds
Bromine (Br)
organobromine compounds
Iodine (I)
organoiodine compounds
More than one type of halogen:
various combinations are possible. A well-known example is:Fluorine (F) and Chlorine (Cl)
chlorofluorocarbon (CFC) compounds
also known, simply as:
chlorofluorocarbons (CFCs)
Haloalkanes can be grouped, named, and described according to the particular halogen that has replaced a hydrogen atom in the corresponding alkane.
For each of the main halogens (F, Cl, Br and I), the generic name for compounds that include that halogen is listed below together with some examples of that type of compound.
Fluorine (F) |
organofluorine compounds |
Examples include:
|
Chlorine (Cl) |
organochlorine compounds |
Examples include:
|
Bromine (Br) |
organobromine compounds |
Examples include:
|
Iodine (I) |
organoiodine compounds |
Examples include:
|
When there is more than one type of halogen:
Various combinations are possible.
Consider the example in which molecules of an organic compound include both fluorine (F) and chlorine (Cl) atoms. In that case:
the generic name of the organic compounds is: |
Examples include:
|
2. Haloalkanes by Structure (which carbon atom is the halogen attached to ?)
Haloalkanes are also grouped, named, and described according to the position in the molecule of the carbon atom(s) to which the halogen atom(s) is / are attached. Obviously there are a huge number of possibilities, especially in the cases of larger molecules. Only the simplest rules are mentioned here.
Primary, Secondary and Tertiary Haloalkanes
Structure
Description
Example
of a molecular structure
Examples of other simple haloalkanes
Primary (1°) haloalkanes
The carbon atom to which the halogen atom is attached is only attached to one other carbon atom (or 'alkyl group' = CH3).
Secondary (2°) haloalkanes
The carbon atom to which the halogen atom is attached is attached to two other carbon atoms, so forms two C-C (single) bonds.
Tertiary (3°) haloalkanes
The carbon atom to which the halogen atom is attached, is attached to three other carbon atoms, so forms three C-C (single) bonds.
1-chloroethane
2-fluoropropane
2-bromo-2-methylpropane
Haloalkanes are also grouped, named, and described according to the position in the molecule of the carbon atom(s) to which the halogen atom(s) is / are attached. Obviously there are a huge number of possibilities, especially in the cases of larger molecules.
Only the simplest rules are mentioned here.
Primary, Secondary and Tertiary Haloalkanes
The three simplest types of haloalkanes by structure are primary (1°), secondary (2°) and tertiary (3°) haloalkanes. Descriptions, examples of a simple structure, and lists of names of some of each of these types of haloalkanes are shown below.
- Primary (1°) haloalkanes
The carbon atom to which the halogen atom is attached is only attached to one other carbon atom (or "alkyl group" = CH3).Example of a molecular structure:
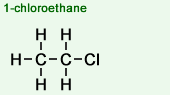 Examples:
Examples:- 1-fluoroethane
- 1-bromobutane
- 1-iodohexane
- 1-fluoropentane
- 1-chloro-methylpropane
- 1-iodo-methylbutane
- Secondary (2°) haloalkanes
The carbon atom to which the halogen atom is attached is attached to two other carbon atoms, so forms two C-C (single) bonds.Example of a molecular structure:
 Examples:
Examples:- 2-chlorobutane
- 3-fluoropentane
- 2-iodoheptane
- 4-chloro-octane
- 2-fluoro-3-methylpentane
- 3-bromo-2-methylhexane
- Tertiary (3°) haloalkanes
The carbon atom to which the halogen atom is attached, is attached to three other carbon atoms, so forms three C-C (single) bonds.Example of a molecular structure:
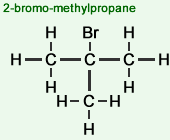 Examples:
Examples:- 2-chloro-2-methylbutane
- 3-fluoro-3-methylpentane
- 3-iodo-3-methylheptane
- 2-fluoro-2-ethylpropane
- 2-fluoro-2-ethylhexane
- 4-iodo-4-methylheptane
| 1-chloroethane | |
| fluoropropane |  |
| 2-bromo-2-methylpropane |  |
Chemistry and Reactions of Haloalkanes (more advanced information)
The chemical reactions of haloalkanes generally involve the halogen part of the molecule (which distinguishes it from the corresponding alkane).
Haloalkanes are polar molecules, meaning that the carbon to which the halogen is attached is slightly electropositive and the halogen is slightly electronegative. This results in an electron deficient (electrophilic) carbon atom which, inevitably, attracts nucleophiles (electron-rich chemical reactants that are attracted by electron deficient compounds). Therefore haloalkanes tend to be reactive with nucleophiles, with which they undergo nucleophilic substitution reactions.
Common reactions of haloalkanes include:
- haloalkane with potassium hydroxide
- haloalkane with potassium cyanide
- haloalkane with ammonia
- haloalkane with a sodium salt of a carboxylic acid
- haloalkane with a alkoxide
- formation of Grignard reagents from haloalkanes
Relative Rates of Reactions:
Generally, iodoalkanes react more rapidly that bromoalkanes, which react more rapidly that chloroalkanes.






















