
How to draw Organic Molecules
Drawing Organic Molecules (in general)
There are several standard ways to represent the structures of organic molecules in different levels of detail.
These include:
- Molecular Formulae of Organic Molecules
- Structural Formulae of Organic Molecules:
- Fully Displayed Formulae
- Simpler Displayed Formulae
- Sketched 3D Formulae
- Modelled 3D Formulae
- Skeletal Formulae
For more about drawing organic molecules generally see: How to Draw Organic Molecules.
Representation of organic molecules can be challenging because:
- Organic molecules can be very large and are sometimes also extremely complicated.
- Isomerism: The same combination of atoms can be attached together in different ways (isomers), resulting in different chemicals that have different properties. For this reason correctly stating the numbers of each type of atom in a molecule of a compound is sometimes insufficient to uniquely identify the compound.
- Bond angles and hence the 3-dimensional structure of organic molecules can also be important.
It is useful to be able to label the different ways of representing (including drawing) organic molecules and to recognise and be able to give examples of each.
Molecular Formulae
Molecular formulae will already be familiar from the short-hand way used to represent chemical compounds in school-level inorganic chemistry.
In organic chemistry a molecular formula just states the numbers of each type of atom present in a molecule, without providing any information about the way those atoms are arranged or joined together. Because they do not include details of the bonding within the molecule, molecular formulae are rarely sufficient in organic chemistry. |
Examples of molecular formulae: |
In organic chemistry a molecular formula just states the numbers of each type of atom present in a molecule, without providing any information about the way those atoms are arranged or joined together.
Because they do not include details of the bonding within the molecule, molecular formulae are rarely sufficient in organic chemistry.
Examples of molecular formulae:
- Propane has the molecular formula: C3H8
- Butanol has the molecular formula: C4H9OH
- Ethanal has the molecular formula: C2H4O
- Ethylamine has the molecular formula: C2H7N
- Propanoyl Chloride has the molecular formula: C3H5ClO
- Octanoic Anhydride has the molecular formula: C16H30O3
When molecular structures are used in organic chemistry it tends to be to describe the reactions of relatively simple molecules, e.g. combustion of hydrocarbons.
C3H8 + 5 O2 |
3 CO2 + 4 H2O |
|
propane + oxygen |
carbon dioxide + water |
|
Where the purpose is obviously to describe a simple reaction rather than to consider the organic molecule itself in any detail.
Structural Formulae
In contrast to molecular formulae, structural formulae do illustrate how the atoms are joined together by various (single, double or triple) chemical bonds.
There are several different types of structural formulae.
They show different levels of detail and types of information, e.g. all the individual bonds (or not), approx. bond-angles (or not), representation in 2-dimensions or 3-dimensions (albeit often on 2D paper or computer screens, but sometimes as physical 3D models).
The different types of structural formulae of organic molecules are listed and defined below, with simple examples of each. Further information about these different representations of the structural formulae of organic molecules is included on more detailed pages about each - see links after each section.
1. Displayed Formulae
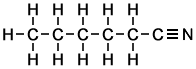
Fully Displayed Formulae are the opposite of simple Molecular Formulae because, while molecular formulae give no information about the molecular structure and bond types, fully displayed formulae include every atom in the molecule identified individually by its atomic symbol and every bond between atoms drawn in full so that it is possible to see which atoms are connected to which other atoms and by which type (single, double or triple) of covalent bond.
Examples of Fully Displayed Formulae
Propanoic Acid |
Hexanenitrile |
Propyl Pentanoate |
|
 |
 |
 |
Propanoic Acid
Hexanenitrile
Propyl Pentanoate
Use of fully displayed formulae is very helpful when first studying organic chemistry and gaining practice working out molecular structures using the rules about how many other atoms each type of atom connects to, which bonds can be double or triple bonds, and so on. However, drawing out fully displayed formulae is not always necessary and can be impractical in the cases of molecules that exceed a certain size and complexity. In such situations simplified displayed formulae and skeletal formulae may be more appropriate.
2. Simplified (Semi-Displayed) Formulae, e.g. incl. CH3
Displayed molecular formulae can be simplified for ease, speed, space and clarity by grouping together the hydrogen atoms attached to each carbon atom in the chain(s). Functional groups attached to the molecule are often still drawn in full as these are important parts of the molecule, frequently responsible for its key properties - including reactions.
Depending on the level of 'simplification', single covalent bonds between adjacent carbon atoms in a chain or branch may, or may not, be shown (see examples below).
Examples of Simplified Displayed Formulae
Note that the molecules represented below are the same as those represented by fully displayed formulae (above).
Propanoic Acid
Hexanenitrile
Propyl Pentanoate
Propanoic Acid |
Hexanenitrile |
Propyl Pentanoate |
|
3. 3-D Structural Formulae (Sketched)
Sometimes it is necessary to show the 3-dimensional (often called simply '3-D' or '3D') arrangements of atoms in organic molecules. Conventional symbols may be used to do this. They are:
A simple example of a 3D Structural Formula is that for methane, whose molecular formula is CH4.
Although it is useful to be able to draw 3D molecular structures of organic compounds, these diagrams can quickly become cumbersome and difficult to draw clearly. You are unlikely to have to draw 3D molecular structures of very complicated molecules for school chemistry tests. A common use of this type of representation of molecular structures is to explain isomerism, and optical isomerism in particular.
See also How to Draw Organic Molecules in 3D.
4. 3-D Structural Formulae using Models, e.g. 'Ball and Stick'
Teaching institutions such as schools, colleges and universities have used 'ball and stick' models to teach and explain organic chemistry for many years. Such learning tools are also available to purchase (online or in academic bookshops) for personal study. More recently computer software has also become available to enable students and professional chemists to create and view computer-generated images of molecular structures that can be rotated in '3D space' on a computer screen or interactive whiteboard. Both the physical and software versions of chemistry 'ball and stick' models are useful for understanding and communicating molecular structures.
Examples of Fully Displayed Formulae vs. 3D Structural Formulae (using models)
Methanol (CH3OH)
Ethylamine (C2H7N)
Methoxy Methane (C2H6O)
Molecule |
3D Sketch of molecule |
'Ball & Stick' 3D Model of molecule |
|
Methanol (CH3OH)
|
|
|
|
Ethylamine (C2H7N)
|
|
|
|
Methoxy Methane
|
|||
See also 3D Models of Organic Molecules (to be added).
5. Skeletal Formulae
In organic chemistry skeletal formulae are the most abbreviated diagrammatic descriptions of molecules in common use. They look very bare because the hydrogen atoms (attached directly to carbons) are not shown, resulting in the appearance of just a 'carbon skeleton' with functional groups attached to it.
This type of representation of organic molecules is most frequently used in more advanced texts, research papers, and specialist areas. It is generally the most practical way to draw large and very complicated organic molecules. Even at lower levels of complexity (e.g. High School Chemistry and UK A-Level) skeletal formulae may be used, especially when studying and describing structures involving carbon rings, such as cycohexane and benzene.
Examples of Skeletal Formulae
Note that the molecules represented below are the same as those represented by fully displayed formulae (above, Section 1) and simplified displayed formulae (above, Section 2).
Propanoic Acid
Hexanenitrile
Propyl Pentanoate
Propanoic Acid |
Hexanenitrile |
Propyl Pentanoate |
|
 |
 |
 |
Note that the hydrogen atoms are present in the molecules but in skeletal formulae their presence is assumed, rather than drawn or stated explicitly.
See also How to draw Skeletal Formulae of Organic Molecules.


















